Industry
Primary Engineering Controls for Drug Compounding
Enhancing Safety and Efficiency in Drug Compounding EnvironmentsNuAire, a leading manufacturer of laboratory equipment, is dedicated to providing primary engineering controls designed for compliance with USP 797 and USP 800 standards. Our state-of-the-art solutions ensure the safety and efficiency of drug-compounding environments, protecting both the personnel and the final product. By incorporating advanced technology and adhering to stringent quality measures, NuAire delivers reliable and consistent performance that meets the critical requirements of today's pharmaceutical compounding industry—Trust NuAire to safeguard your compounding processes and elevate your facility's compliance standards.

Industry
Primary Engineering Controls for Drug Compounding
NuAire, a leading manufacturer of laboratory equipment, is dedicated to providing primary engineering controls designed for compliance with USP 797 and USP 800 standards. Our state-of-the-art solutions ensure the safety and efficiency of drug-compounding environments, protecting both the personnel and the final product. By incorporating advanced technology and adhering to stringent quality measures, NuAire delivers reliable and consistent performance that meets the critical requirements of today's pharmaceutical compounding industry—Trust NuAire to safeguard your compounding processes and elevate your facility's compliance standards.
Pharmacy PEC
To help you with the compounding process, NuAire has created a range of pharmacy equipment that meets the U.S. Pharmacopeia's <797> standard for compounding sterile preparations and U.S. Pharmacopeia's <800> standard Hazardous Drugs—Handling in Healthcare Settings. You can rely on NuAire's powder weight stations to accurately and safely weigh powders and particles, even if they are hazardous. You can use NuAire's primary engineering controls, such as a compounding aseptic isolator (CAI), which provides a sterile, positive pressure work environment for compounding non-hazardous drugs, or a compounding aseptic containment isolator (CACI), which provides a sterile, negative pressure work environment that minimizes exposure to airborne toxins and other hazardous materials. NuAire also has biosafety cabinets (BSC), laminar airflow workstations (LAFW), containment ventilated enclosures (CVE), and custom robotic enclosures for use in a compounding pharmacy.
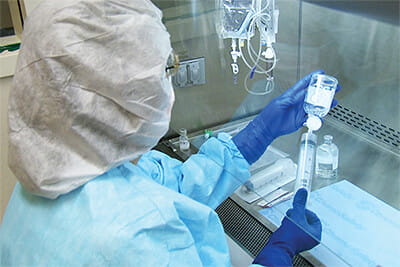
application
Sterile Hazardous Drug Compounding C-PEC
NuAire provides containment primary engineering controls (C-PEC) for the safe handling of sterile hazardous drug compounding for USP 800 compliance.

Application
Sterile Non-Hazardous Drug Compounding PEC
Choose laminar airflow workstations (LAFW) or compounding aseptic isolators (CAI) for sterile non-hazardous drug compounding for USP 797 compliance.

Application
Non-Sterile Hazardous Drug Compounding C-PEC
Safely and effectively compound non-sterile hazardous drug powders in a containment ventilated enclosure (CVE) for USP 800 compliance.

Application
Medication Workflow
Maximize your medication workflow solution in a primary engineering control (PEC) or containment primary engineering control (C-PEC) designed for scales, barcode scanners, computers, and more.
Sterile Non Hazardous Compounding Products
Sterile Hazardous Drug Compounding Products
PharmaGard
NU-NR800
PharmaGard







