
 Webinar
Webinar
USP Compliance USP 795 Facility Requirements
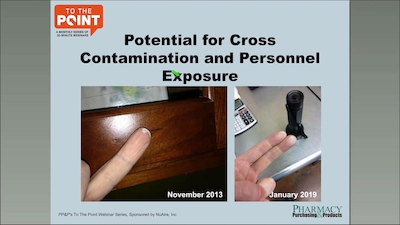

Bryan Prince reviews the timeline for USP 795 compliance, QC processes for non-sterile compounding, staff training steps, and more.
Original content from Pharmacy Purchasing & Products - www.pppmag.com

