

Credentials and Affiliations
Certifications

About USP 800 Expert Bryan Prince
Who is Bryan Prince?
Bryan Prince, MBA is a USP <800> and compounding workflow consultant and Senior Lab Planner and Compounding Pharmacy Specialist at Ci Design, Inc. He helps pharmacies and laboratories design safe, efficient, and compliant compounding spaces.
What does Bryan specialize in?
Bryan specializes in hazardous drug handling, engineering controls, cleanroom and facility design, and compounding workflow strategies that support USP <800> and related guidelines.
How does Bryan work with NuAire?
Bryan collaborates with NuAire as a subject matter expert, author, and speaker. He develops videos, white papers, and webinars that explain how to use primary engineering controls and cleanroom designs to support safe and compliant compounding.
More by This Author


Primary Engineering Control Placement in the Cleanroom
This white paper explores PEC selection and placement strategies to meet USP <797> and <800> guidelines in cleanrooms handling sterile and hazardous drug compounding.
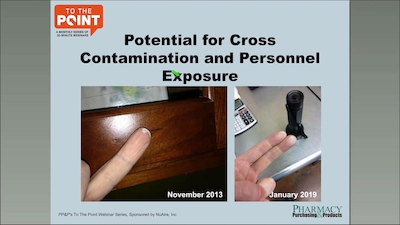

USP Compliance USP 795 Facility Requirements
Bryan Prince reviews the timeline for USP 795 compliance, QC processes for nonsterile compounding, staff training steps, and more.


Working Safely in Your Containment Ventilated Enclosure
Training video on working safely in a Containment Ventilated Enclosure (CVE): PPE, staging, airflow technique, wet-transfer methods, waste handling, and cleaning to reduce exposure and contamination.


PEC and C-PEC Installation Process
Discover the intricacies of installing Primary Engineering Controls (PECs) and Containment Primary Engineering Controls (C-PECs) in a pharmacy setting through our insightful white paper authored by Bryan Prince.


Improve Workflow with Cleanroom Design
<p>This white paper discusses 503A pharmacy requirements and USP chapters <797> and <800> as it relates to cleanroom design.</p>


Facility and Engineering Controls Using USP 800 Guidelines
The focus of this article is to discuss the details for consideration of a good working design of a USP 800 compliant compounding lab.


Facility and Engineering Control Design Under USP 800
Design considerations for creating a Pharmacy USP 800 compliant Hazardous Drug (HD) compounding operation.


Design Tomorrow's Drug Compounding Cleanroom Today
This white paper covers design considerations to keep in mind when designing your drug compounding cleanroom for the future.
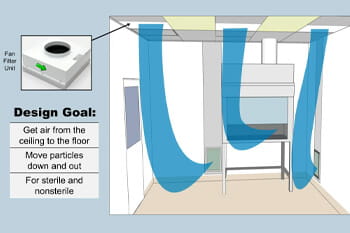

Engineering Control Options Under USP 800
Explore USP 800 engineering controls in our webinar with expert Bryan Prince. Learn about air handling and facility design for pharmacy safety.


Budgeting for USP 800
Budget considerations for cleanroom construction. Weigh the options between new construction versus remodeling, and other dilemmas that hospital pharmacies may face preparing for USP 800 compliance.


