White Paper
White Paper
Understanding Compounding Isolators


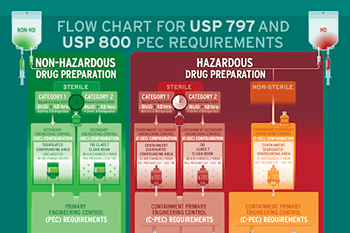
Safety for staff and patients is the primary goal when handling and compounding drugs in the pharmacy. Conducting aseptic processing with isolation systems separates the cleanroom environment from the aseptic processing line and minimizes exposure to personnel. Per US FDA’s current good manufacturing practice (cGMP) guidance documents, a well-designed positive pressure isolator, supported by proper maintenance and monitoring, increase safety by preventing opportunities for microbial contamination during compounding.