Article
Article
How a Class II Type A2 BSC Works

Perhaps you’ve wondered what is a biological safety cabinet and wondering about a BSCs uses or application settings. The NuAire Class II, Type A2 Biosafety Cabinet (BSC) stands at the forefront of design and functionality, crucial for creating clean, controlled environments to advance scientific research. Manufacturers, including NuAire, construct these cabinets to align with minimal standards such as NSF/ANSI 49 and EN 12469. Independent parties test these standards to ensure the cabinets can be officially listed. This process guarantees that the cabinets meet the highest safety and functionality standards, making them indispensable in research and clinical settings worldwide.
Protection Offered
The design of Class II, Type A2 BSCs offers comprehensive protection:
- Personnel Protection: Inward airflow prevents hazardous substances from escaping and reaching laboratory personnel.
- Product Protection: HEPA-filtered laminar airflow over the work surface minimizes cross-contamination between samples.
- Environmental Protection: Exhaust air is filtered before release, reducing hazardous substance emissions.
These protection mechanisms are vital in ensuring that laboratory personnel are not exposed to potentially harmful biological agents, that the integrity of the work being conducted within the cabinet is maintained, and that no contaminants are released into the environment. This triple protection is essential for maintaining the high standards of safety required in modern laboratories.
What’s A Biological Safety Cabinet? | Airflow Dynamics and Protective Mechanisms
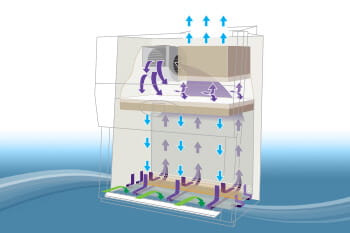
The effectiveness of the Type A2 BSC starts with its sophisticated airflow management. It draws room air through the front grille, creating a protective air curtain to prevent hazardous aerosols from escaping the cabinet. This inflow air acts as a barrier at speeds of at least 100 feet per minute (0.51 m/s), ensuring personnel protection against biohazards.
The cabinet channels air under the work surface and up through a rear plenum powered by an internal blower. This air is cleverly divided: approximately 70% is recirculated through a HEPA filter back into the cabinet as laminar, or unidirectional, downflow over the work area. This airflow pattern minimizes the potential for cross-contamination, providing stringent product protection necessary for good manufacturing processes.
What’s A Biological Safety Cabinet? | HEPA Filtering
A dedicated HEPA filter exhausts the remaining 30% of the contaminated air from the cabinet. This feature underscores the environmental protection these cabinets provide, ensuring no contaminants escape into the laboratory or external surroundings.
HEPA filters are critical components in the airflow dynamics of BSCs. HEPA, which stands for High-Efficiency Particulate Air, filters are designed to trap 99.99% of particles that are 0.3 microns in diameter. This level of filtration is essential for maintaining the contaminant-free conditions required in many laboratory applications, from handling hazardous pathogens to preparing sterile pharmaceutical compounds.
What’s A Biological Safety Cabinet? | Independent Functionality and Laboratory Integration
A vital attribute of the Type A2 BSC is its capability to operate independently from a laboratory's HVAC system. It features a motor or motors that manage airflow to maintain precise and safe conditions inside the cabinet. This independence allows for more flexible laboratory design and reduces installation costs by eliminating the need for external ducting. When handling small quantities of volatile chemicals, the BSC can be optionally connected to the building's exhaust system via a canopy connection, enhancing safety by effectively removing hazardous chemical vapors from the work environment.
Integrating BSCs into laboratory environments must consider several factors. These include the layout of the laboratory, the types of activities being performed, and the need for flexibility in the use of space. By providing a self-contained environment, Type A2 BSCs allow for greater adaptability in laboratory design, which can be particularly important in settings where space is at a premium or where activities may change over time.
Additionally, the ability to connect the BSC to the building's exhaust system when necessary adds an extra layer of safety. This is particularly important when the BSC is used for procedures that generate hazardous chemical vapors. The canopy connection ensures that these vapors are effectively removed from the laboratory, protecting personnel and the environment from potential exposure.
What’s a Biological Safety Cabinet? | Global Standards
Class II, Type A2 cabinets are developed by manufacturers to align with the rigorous requirements of international safety standards, targeting certification under NSF/ANSI 49 and EN 12469. These standards are the most widely recognized benchmarks for modern-day biosafety cabinets, setting guidelines for structural integrity, airflow dynamics, and filtration efficacy critical for certifying a BSC's safety capabilities. NSF/ANSI directly manages listings and certifications for compliance with its standards, while certifications under EN 12469 are handled by third-party certifiers such as TÜV Nord. Achieving these certifications is a testament to a product's compliance and reliability across global laboratories.
NSF/ANSI 49 sets the standard for biosafety cabinet design, construction, performance, and field certification in the United States. This standard includes rigorous testing for airflow velocity, HEPA filter integrity, and cabinet leakage. Cabinets that meet these standards ensure safety and performance, which are critical in laboratory settings where the stakes for contamination are high.
EN 12469 is the European standard for microbiological safety cabinets. It sets out requirements for the design, construction, and performance of BSCs, similar to NSF/ANSI 49. Cabinets that achieve certification under EN 12469 are recognized for their ability to protect personnel, products, and the environment from biohazardous materials. Third-party certification by organizations like TÜV Nord provides additional assurance of compliance with these rigorous standards.
What’s a Biological Safety Cabinet? | Applications in Diverse Laboratory Settings
Biological safety cabinets are indispensable across diverse settings, including pharmaceutical development, clinical research, microbiology laboratories, and pharmacy drug compounding. They are essential for applications involving pathogens or sterile materials, where rigorous control of cross-contamination is crucial.
Together with NuAire’s Ultra Low Temperature Freezers, Class II, Type A2 BSCs in tissue culture labs provide an aseptic environment for manipulating sensitive samples that protect cultures from environmental contaminants. Similarly, in pharmacy settings, these cabinets ensure the sterility of compounded medications, safeguarding pharmaceutical preparations from microbial contamination and environmental pollutants—critical for patient safety and product efficacy.
In pharmaceutical development, Biological safety cabinets maintain clean air conditions for formulating new drugs. This is critical for ensuring the efficacy of the drugs and protecting the health of the patients who will eventually use them. Any contamination during the development process could compromise the safety and effectiveness of the drug, potentially leading to serious health consequences.
Clinical research laboratories rely on Biosafety cabinets to protect both personnel and the integrity of their research. Whether working with infectious agents or conducting sterile cell culture experiments, researchers must ensure that external contaminants do not result in cell culture contamination. The use of BSCs provides the necessary level of protection to maintain the validity of experimental results, which is crucial for advancing scientific knowledge.
Microbiology laboratories use BSCs to handle various biohazardous materials. From studying bacteria and viruses to working with genetically modified organisms, microbiologists require environments that protect themselves and their samples. Class II, Type A2 BSCs provide the necessary containment to safely conduct these studies, minimizing the risk of accidental exposure or contamination.
Pharmacy drug compounding involves preparing personalized medications for patients, often involving aseptic techniques. BSCs are essential in these settings to prevent microbial contamination of the medications. This is particularly important for patients with compromised immune systems or those receiving intravenous medications, where even minor contamination could lead to serious infections.
What’s a Biological Safety Cabinet? | Technological Advancements and Ergonomic Features
Modern Class II, Type A2 BSCs incorporate advanced technologies to enhance their functionality and ease of use. For instance, many models now feature energy-saving ECM motors, which offer improved efficiency and reduced operational costs. These motors provide greater torque, extending the lifespan of HEPA filters and reducing the frequency of filter changes. Additionally, ECM motors generate less heat, decreasing the load on laboratory HVAC systems and contributing to a more comfortable working environment.
The incorporation of advanced control systems, such as the FlowGard™ control system, allows for precise monitoring and adjustment of airflow and other critical parameters. These systems often include features such as LED displays, touchscreens, and alarm systems that alert users to any deviations from optimal operating conditions. Such advancements ensure the BSC operates within safe parameters, providing continuous protection for personnel and products.
Ergonomic design is another important consideration in developing modern BSCs. Features such as adjustable work surfaces, comfortable armrests, and optimized viewing windows are designed to reduce physical strain on users. This is particularly important in laboratory settings where personnel may spend long hours working within the cabinets. By improving comfort and reducing the risk of repetitive strain injuries, ergonomic designs contribute to the well-being of laboratory personnel and the overall efficiency of laboratory operations. Ergonomically-effective BSCs are a key component of good lab design.
What’s a Biological Safety Cabinet? | Maintenance and Certification
Regular maintenance and certification of Class II, Type A2 BSCs are essential to ensure their safe operation. Certification typically involves testing by qualified technicians to verify that the cabinet meets all relevant standards for airflow, HEPA filter integrity, and overall performance. This process may include tests for inflow and downflow velocities, leak tests on the HEPA filters, and checks for any physical damage to the cabinet. Proper maintenance and HEPA air filter use extends the life of your BSC.
Maintenance tasks often include replacing HEPA filters, which should be done according to the manufacturer's recommendations or when performance tests indicate a decline in efficiency. Other routine maintenance activities include cleaning and disinfecting the cabinet's interior surfaces, checking the control systems' operation, and ensuring that all seals and gaskets are intact and functioning properly. Regular maintenance helps extend the BSC's lifespan and ensures that it continues to provide the necessary level of protection.
What’s A Biological Safety Cabinet? | An Investment In Safety and Precision
The Class II, Type A2 Biosafety Cabinet ensures laboratory safety and precision across various scientific and pharmaceutical settings. These cabinets embody the convergence of advanced engineering, stringent standards compliance, and practical functionality. Implementing Type A2 BSCs in laboratories not only underscores a commitment to maintaining clean conditions but also enhances the reliability of results, thus contributing significantly to scientific advancements and public health safety.
By effectively managing airflow to provide triple-layered protection—personnel, product, and environmental—these cabinets establish themselves as indispensable tools to handle biohazards safely. As research continues to push the boundaries of what is possible, the role of the Class II, Type A2 Biosafety Cabinet will remain pivotal in navigating the complex landscape of laboratory safety and contamination control.
Need More Information About NuAire Biological Safety Cabinets?
Explore NuAire FAQs as well as resources on purchasing a biological safety cabinet, biosafety cabinet classes, biological safety cabinet construction.
Got a question we haven’t answered? Use our contact us form, we are happy to answer your questions or help you request a quote for a NuAire biological safety cabinet.
Bibliography
- NSF International. NSF/ANSI 49 - Biosafety Cabinetry: Design, Construction, Performance, and Field Certification. 2022.
- EN 12469: Biotechnology – Performance Criteria for Microbiological Safety Cabinets. European Committee for Standardization (CEN), 2000.
- Centers for Disease Control and Prevention (CDC). Biosafety in Microbiological and Biomedical Laboratories (BMBL), 5th Edition. 2009.
- American Biological Safety Association (ABSA). ABSA International Guidelines and Standards.
- International Federation of Biosafety Associations (IFBA). IFBA Certification Program.
- World Health Organization (WHO). Laboratory Biosafety Manual, 4th Edition. 2020.