Webinar
Webinar
Engineering Control Options Under USP 800

The webinar "Engineering Control Options Under USP 800," facilitated by Bryan Prince, a seasoned USP 800 design consultant, offers a comprehensive guide on the best practices for engineering controls in pharmacy settings compliant with USP 800 standards. Bryan's expertise stems from a decade in the building industry before transitioning to pharmaceutical-engineered containment, making him a trusted voice in chemical handling and safety strategies.
USP 800 sets rigorous standards for handling hazardous drugs in healthcare settings to protect pharmacy staff and prevent contamination. Engineering controls are central to these regulations, ensuring that environments where hazardous drugs are prepared maintain stringent contamination controls.
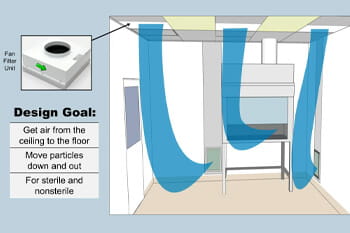
Bryan outlines the classifications within USP guidelines—USP 795, USP 800 sterile, and non-sterile. For instance, USP 795 areas utilize standard HVAC systems, while USP 800 areas require robust air management systems to maintain negative pressure and prevent the recirculation of contaminated air.
One critical takeaway from the webinar is the necessity of dedicated makeup air units for USP 800 non-sterile and sterile environments. These units are essential for achieving air changes per hour (ACH), a necessary standard in maintaining safe compounding practices. Bryan stresses the importance of planning for higher ACH than the minimum to accommodate additional heat loads from equipment and personnel, which can affect air handling system performance.
The webinar also dives into the spatial design and how pharmacy layout should facilitate workflow while adhering to safety standards. Bryan introduces the concept of airlocks and clean corridors to manage different pressure zones within the pharmacy, ensuring that contamination risks are minimized.
The discussion extends to the practical aspects of installing engineering controls, such as selecting and placing HEPA filters, configuring ductwork, and considerations for
makeup air units. Bryan emphasizes the need for a custom approach to each pharmacy's design to meet the specific requirements of USP 800, which often includes constructing new air handling systems rather than modifying existing ones.
Bryan's insights are precious when he discusses the challenges of retrofitting older pharmacies to meet new standards. He highlights real-world scenarios where pharmacies had to overhaul their HVAC systems to comply with the stringent demands of USP 800, underscoring the complexities involved in ensuring that these systems can handle the specified air volumes and pressures.
In conclusion, Bryan reiterates the importance of collaboration between pharmacy staff, architects, and engineers to create environments that are not only compliant with USP 800 but also conducive to efficient and safe work practices. The webinar is a crucial resource for anyone involved in the design, renovation, or operation of facilities handling hazardous drugs, providing them with the knowledge to make informed decisions about engineering controls under USP 800.
Original content from Pharmacy Purchasing & Products