White Paper
White Paper
Standard Operating Procedures (SOPs) for Biosafety Cabinet Use


Why SOPs Matter in Biosafety Cabinet Operation
Creating written standard operating procedures (SOPs) for using a biosafety cabinet is critical to maintaining lab safety, compliance, and research integrity. While many laboratories have technical SOPs for experiments, documenting BSC use ensures biological containment and minimizes exposure risks. This white paper outlines why BSC SOPs are essential components of a strong biosafety program.
Key Procedures That Require SOPs
The white paper details three key areas where written SOPs should be developed for BSC work:
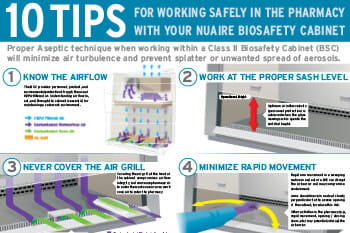
- Setting up and using the BSC: Learn best practices such as working clean-to-dirty, managing airflow, and collecting waste, based on risk mitigation guidance found in resources like biosafety cabinet risk assessments.
- Cleaning and decontaminating the BSC: Understand surface decontamination processes and when deeper maintenance is required. More tips are available in our guide on surface decontamination considerations.
- Responding to emergencies: Learn how to handle spills, alarms, or power failures inside the BSC, building on best practices discussed in our spill containment article and alarm response guide.
Effective SOP Writing and Implementation
Developing SOPs that are easy to understand and follow is crucial for laboratory personnel. This white paper shares tips for drafting, approving, distributing, and regularly reviewing BSC SOPs, ensuring they remain up-to-date and effective over time. Incorporate training initiatives like those suggested in our article creating a biosafety cabinet training program to build a comprehensive biosafety culture.
Enhance Your Lab’s Biosafety Strategy
Whether you're setting up a new facility or improving an existing lab program, having well-written SOPs is vital. Coupled with proper field certification of biosafety cabinets and informed biosafety cabinet selection, standardized practices help maintain optimal lab function and personnel safety.
Download the Full White Paper
Ready to create or refine your biosafety cabinet SOPs? Our detailed guide offers actionable advice, real-world examples, and expert insights to strengthen your lab’s biosafety program.
Get the tools you need to develop safe, compliant, and effective SOPs for your biosafety cabinet operations. Download the full white paper today!